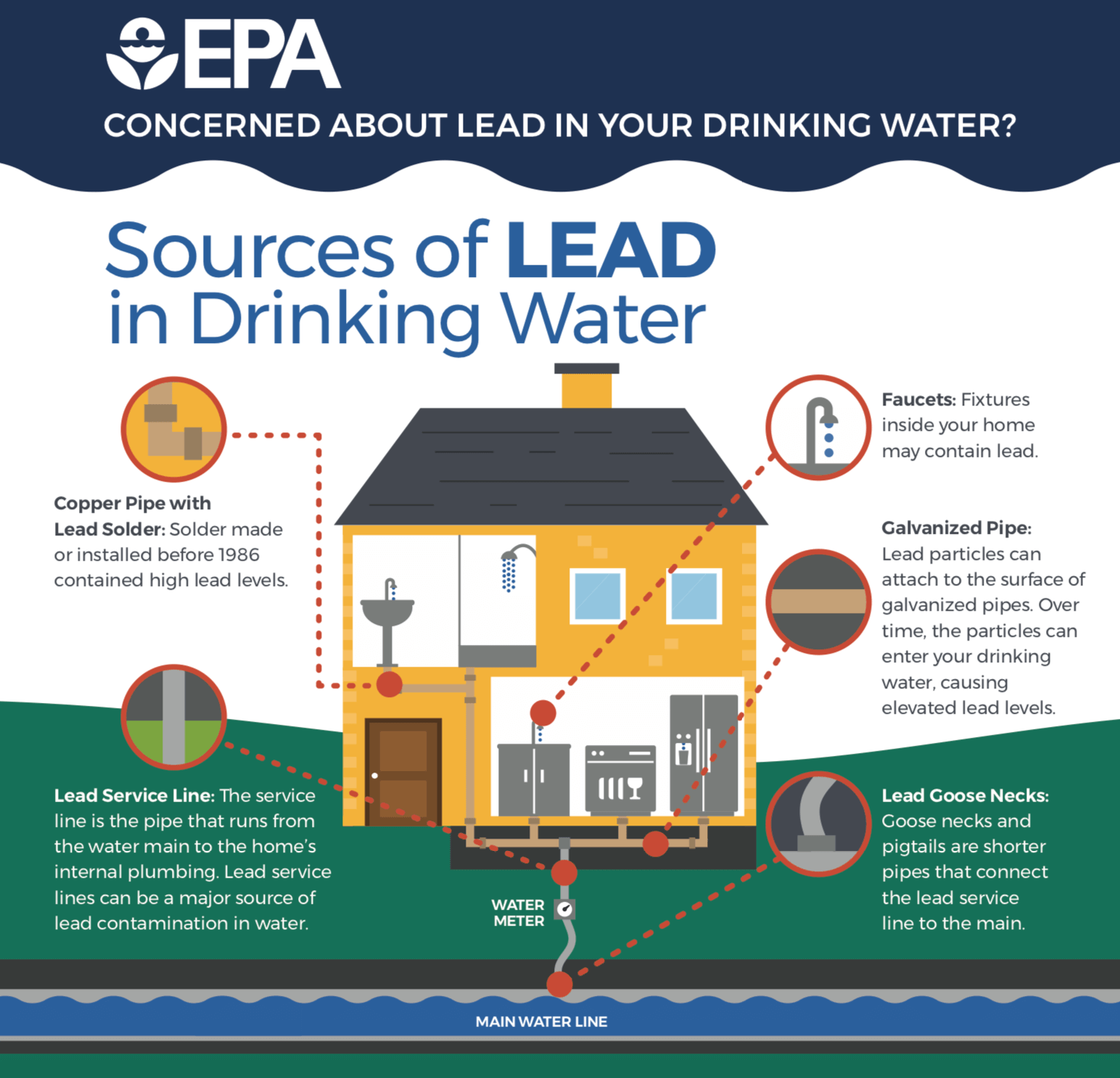
One of GGI’s six focus areas is providing students access to clean drinking water. One such contaminate is lead – a serious issue for health, and it can happen anywhere.
In order to protect students from this health hazard, you must first understand how lead contamination happens in the first place.
 According to the United States Environmental Protection Agency,
According to the United States Environmental Protection Agency,
“Lead can enter drinking water when plumbing materials that contain lead corrode, especially where the water has high acidity or low mineral content.”
Factors that can cause lead to enter our water include:
- Acidity and alkalinity of the water.
- Types and amounts of minerals in the water.
- Amount of lead the water comes into contact with.
- Temperature of the water.
- Wear/corrosion in the pipes.
- How long the water stays in pipes, and
- The presence of protective scales or coatings inside the plumbing materials.
The Safe Drinking Water Act requires the EPA to determine the level of contaminants in drinking water at which no adverse health effects are likely to occur with an adequate margin of safety. In reference to the bullet points above, drinking water is considered lead-free if it contains less than 0.25 percent of lead across all wetted surfaces of pipes, pipe fittings, plumbing fittings, and fixtures.
The EPA shares that children in particular are highly susceptible to lead poisoning.
“A dose of lead that would have little effect on an adult can have a significant effect on a child.”
Health and cognitive issues in children is a key reason to care about lead. Examples include:
- Behavior and learning problems
- Lower IQ and hyperactivity
- Slowed growth
- Hearing problems
- Anemia
- In rare cases: seizures, coma and even death.
Signs of lead poisoning or exposure in older children and adults are:
- Cardiovascular effects, increased blood pressure and incidence of hypertension
- Decreased kidney function
- Reproductive problems (in both men and women)
Now that we understand how lead contaminates water, and why we should care, here’s how schools can prevent their students from lead contamination in their drinking water.
Our CEO Jill Buck recently presented to the Texas PTA at a clean drinking water conference and reported that,
“audience members were pretty disappointed to learn that, despite all we know about the toxins I discussed, they are still perfectly legal and not required to be monitored in all schools in the U.S.”
Although certain levels of dangerous contaminants are legal, there are many things schools can do to limit levels of said toxins.
The first step is to find out about your school’s lead levels in water.
- First and foremost, schools need to have their water tested. You cannot see, taste, or smell lead dissolved in water, so testing is the only sure way of telling whether there are harmful quantities of lead in your drinking water.
- The CDC has created a great resource specifically designed for schools to increase access to lead-free drinking water. This toolkit offers information on lead contamination, water access plans, and a checklist to easily follow and make sure water is safe for students to drink.

At GGI, we have worked with schools in need of clean drinking water and installed state of the art hydration stations, that filter out lead and other contaminants at the point of consumption. Students can use these stations as a water fountain or as a reusable water bottle filling feature.
We believe all students deserve the right to clean, fresh, drinking water at school.
The bottom line: Learning problems, reproductive disorders and organ damage should not be byproducts of attending school.
For additional resources on Lead in Water, and how to eliminate exposure, check out the EPA: